We’ve heard a lot about glucagon-like peptide 1 (GLP-1) receptor agonists lately, primarily via the brand names Ozempic and Wegovy. These types of medications can help manage blood sugar levels, however early research is showing GLP-1 and anesthesia can create adverse complications for the patient. But let’s first go back briefly to the origin.
Enter the Gila monster, a lizard native to the southern United States. A hormone in its venom is like a GLP-1 receptor agonist (GLP-1RA) as it regulates blood sugar levels and modulates appetite by triggering insulin release. This hormone, Exendin-4, optimizes the utilization of the lizard’s food given its sporadic eating, and also helps during hibernation. In 1990, a Canadian endocrinologist first isolated Exendin-4 from a Gila monster’s saliva. Exendin-4 was ultimately synthesized to Exenatide, a GLP-1RA with a half-life sufficient to permit clinical use in treating type 2 diabetes, the first in a new generation of drugs that followed.

Table of Contents
- Mechanism of action of GLP-1
- The emerging clinical research and drug marketing
- Wegovy and Ozempic, side-by-side
- Side effects and adverse events
- Current anesthesia care guidance on patient care
- Summary of where we are
Mechanism of action of GLP-1
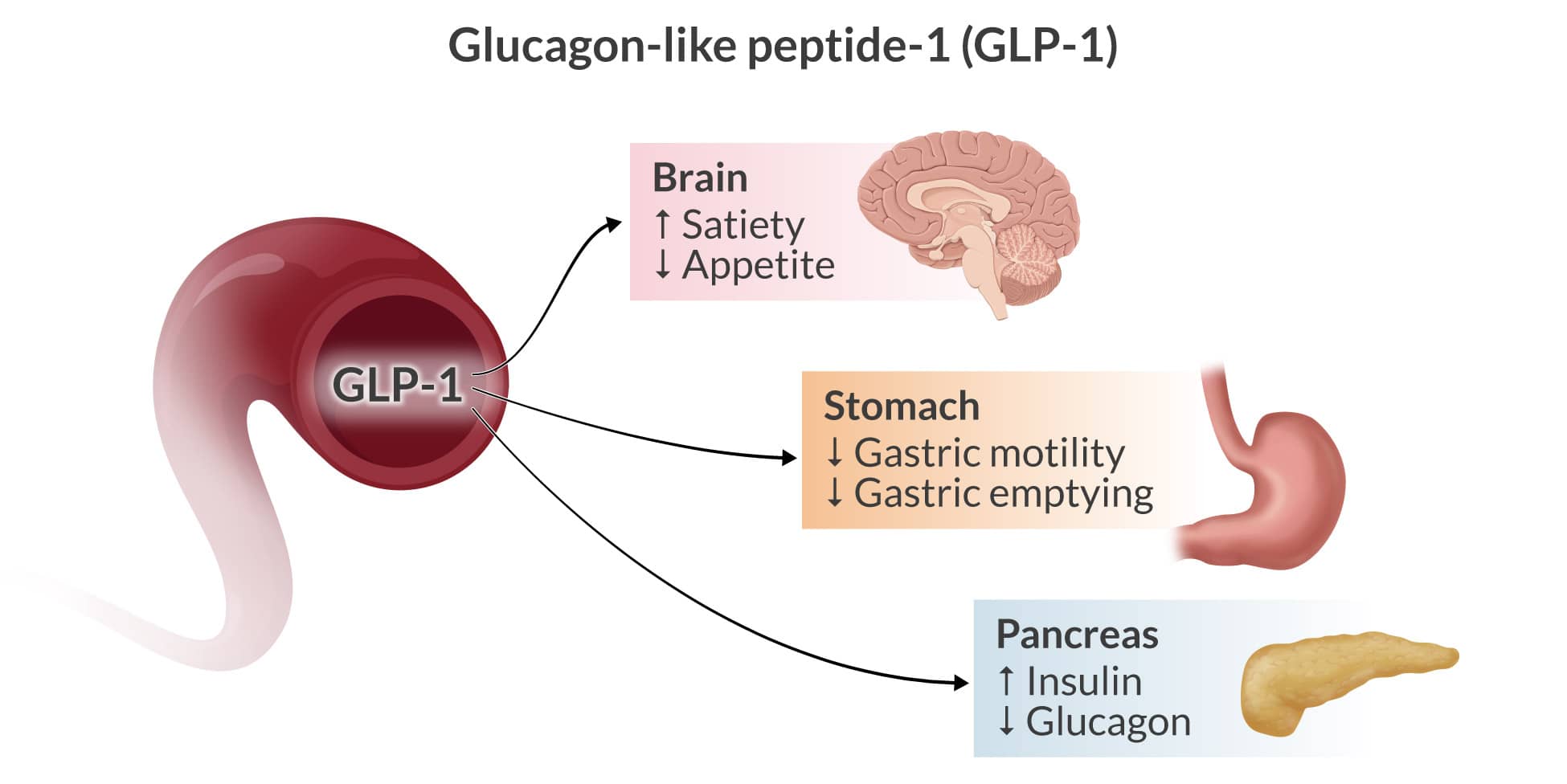
GLP-1 is a hormone inactivated by the enzyme DPP-4. After glucose is ingested, it stimulates insulin secretion; in type 2 diabetes, this is blunted or absent, but GLP-1 can revive insulin secretion. It also delays gastric emptying, inhibits glucagon release from pancreatic alpha cells, causes the proliferation of beta cells, and decreases their apoptosis. It also generates an appetite-suppressant effect. All have clinically valuable targets in the treatment of type 2 diabetes.
Remarkably, there is evidence that GLP-1 agonists can enhance left ventricular ejection fraction, inotropy, coronary blood flow, cardiac output, and vascular endothelial function because of their cardiovascular effects and decrease overall cardiovascular event risks.
The emerging clinical research and drug marketing
An exquisitely performed network meta-analysis of 76 trials investigated the effectiveness of 15 GLP-1RAs in achieving glycemic control, weight loss, and lipid profile in type 2 diabetic adults. Tirzepatide (brand name Mounjaro) resulted in the most significant reduction in hemoglobin A1C, glycemic control, and fasting glucose concentration. An overall benefit of the family of GLP-1RAs was in weight management. While the GLP-1RA drugs have traditionally been used in type 2 diabetics, their ability to cause weight loss has captured the minds and imaginations of patients, providers, and drug manufacturers alike.
The GLP-1RAs are achieving “blockbuster” status, meaning a drug that generates yearly sales exceeding one billion dollars for a drug company. In what amounts to a surprise move, the Center for Medicare and Medicaid Services announced that it will now permit coverage for one of the blockbusters, Wegovy, if prescribed to prevent cardiovascular disease.
In the high-stakes, competitive marketplace for patients and drug manufacturers alike, the available clinical trials have led to the approval of Wegovy as a popular weight-loss drug ahead of Ozempic, another GLP-1RA. Both drugs contain semaglutide, with Ozempic containing a smaller amount of the agent. The marketing of the drugs is being attended by exemplars like “breakthrough drugs” and “game changers,” emotive language swayed in part by the number of people who might use the drugs and the requirement that they be used chronically.
Wegovy and Ozempic, side-by-side
A few caveats about the drugs follow, but keep in mind with the marketplace driving much of the thinking, and clinical experience evolving, there may be changes in store:
- Wegovy is FDA-approved for chronic weight loss management in adults 18 years of age or older with a BMI of 30 or BMI 27 and at least one medical condition related to obesity (e.g., diabetes, hypertension, etc.).
- Ozempic is FDA-approved for the control of blood sugar in type 2 diabetes and decreasing the risk of major cardiovascular events, including death, in those with type 2 diabetes.
- Some prescribe Ozempic off-label for weight management.
- Both drugs are administered once a week with a sub-Q injection. The Wegovy injection pen has a needle already built-in to the device, while the Ozempic pen comes with needles to be attached.
- There have been shortages of the drugs; the demand is very high.
- The cost for each drug can easily exceed $1000 per month.
Side effects and adverse events
GLP-1RA seems to promise—and we are likely to see aggressive marketing in the near term—a sea of change in weight loss management. These agents have both hormonal and brain targets. That said, when we attempt to pharmacologically interfere with, preserve, or augment the body’s innate physiological processes, there are bound to be side effects and adverse events. Such is the case with the GLP-1RAs. Several have significant considerations for patients undergoing anesthesia and surgery.
Below is a table we’ve created for you regarding the more commonly used GLP-1RAs today.
Profiles of the Common GLP-1 Receptor Agonists
| GLP-1RA generic *brand name |
Dosing Approach | Half-life (all renal excreted) | Notes |
| Exenatide
*Byetta, Bydureon |
Sub-Q
Weekly or daily |
3 hours | Thrombocytopenia 2o to immune system trigger |
| Lixisenatide
*Adlyxin |
Sub-Q
Daily |
3 hours | It may no longer be available in the U.S. |
| Semaglutide
*Wegovy, Ozempic *Rybelsis |
Sub-Q
Weekly Oral, daily |
7 days
7 days |
Wegovy is FDA approved for weight loss |
| Liraglutide
*Saxenda, Victoza |
Sub-Q
Daily |
12.5 hours | FDA approved for weight loss |
| Dulaglutide
*Trulicity |
Sub-Q
Weekly |
4.5 days | |
| Tirzepatide
*Mounjaro |
Sub-Q
Weekly |
5 days | FDA approved for weight loss. Also GIP agonist |
Note: All may cause significant/severe GI symptoms including nausea, vomiting, retching, gastroparesis, abdominal bloating, and pain.
Current anesthesia care guidance for diabetic patients taking GLP-1 agonists
Let’s examine the 2023 consensus-based practice guidelines published by the ASA regarding the anesthetic considerations of the GLP-1RAs:
- GLP-1 agonists are associated with many adverse effects, such as nausea, vomiting, delayed gastric emptying, and abdominal pain and bloating.
- Effects on gastric emptying are significant and appear to lessen with long-term use, possibly due to tachyphylaxis of vagal nerve activation.
- Recent reports raise very serious concerns that delayed gastric emptying from GLP-1 agonists increases the risk of regurgitation and aspiration during general anesthesia and deep sedation care.
- Nausea, vomiting, dyspepsia, or abdominal distension in those taking GLP-1 agonists predict increased residual gastric contents.
- Reports of concerns in pediatric patients are overwhelmingly in the 10-18 age range, and mirror those of adults.
- Evidence to provide guidance for anesthesia clinicians is currently sparse.
- Given the considerable concerns the following is suggested:
- Urgent or emergent procedures: treat as a full stomach.
- Elective procedures: if daily dosing, DC on the day of surgery. If weekly dosing, consider holding a week before the procedure.
- If used for diabetes care, consult with an endocrinologist to possibly bridge therapy to minimize the risk of hypoglycemia.
- If significant GI symptoms are present on the day of surgery (nausea, vomiting, bloating, abdominal pain, etc.), consider delaying the procedure.
- If no GI symptoms and GLP-1RAs have been DC’d then proceed.
- If there are no GI symptoms but the drug was not DC’d as suggested, treat as full stomach, evaluate contents with ultrasound, and consider delaying the procedure.
- There currently is no evidence suggesting an optimal fast period.
Following the publication of the guidelines, criticism and questions have emerged. For example, a 2024 paper in Anesthesiology noted:
- Is there any evidence that the risk of gastroparesis or gastric contents is altered by holding the daily or weekly dose? Thus, is it reasonable to “proceed as usual,” as the guidance states?
- Recent literature is cited on patients undergoing upper endoscopy with no predictability regarding holding the drug and finding retained gastric contents.
- Despite suggestions that the gastric emptying effects are greatest in the first 12 weeks and that the effect wanes weeks later, there is no certainty, and the impact on a given patient might be variable.
- Some suggest waiting at least 5 half-lives after holding the drug, which can mean days to weeks, depending on the formulation the patient uses.
- Many recent case reports reveal patients taking GLP-1 drugs having substantial gastric contents even after many hours of fasting.
- Perhaps the better approach is to use an algorithm that includes gastric ultrasound, rapid sequence induction if GA is required, and greater use of clinical signs.
- Writers advise that the ASA guidelines should strongly emphasize the current absence of sufficient evidence.
Summary of where we are on GLP-1 and anesthesia
The highly regarded Anesthesia Patient Safety Foundation has taken a strong stance on the issues involving GLP-1RAs, advising considerable caution on our part when caring for patients using the drugs. The benefits of this family of medications in obese and diabetic patients are acknowledged. However, there are considerable risks, demonstrated by clinical reports, of unappreciated high volumes of gastric contents even when fasting guidelines are followed.
The bottom line is that CRNAs must be hyper-vigilant in caring for patients prescribed GLP-1RAs. According to the ASA and AANA Closed Claims Projects, pulmonary aspiration of gastric contents is one of the top three serious, sometimes catastrophically adverse events that occur during anesthesia care. While guidelines may be of value, CRNAs should have an extremely high level of suspicion and proceed conservatively in these cases until more high-quality evidence emerges.
As CRNAs ourselves, we understand the challenge of fitting CRNA continuing education credits into your busy schedule. When you’re ready, we’re here to help.







