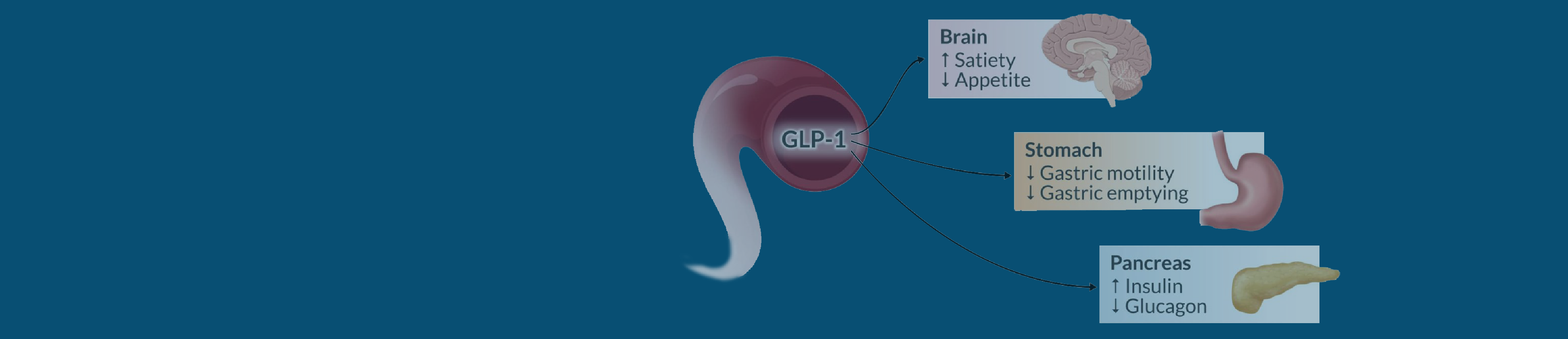
GLP-1 receptor agonists are making headlines. From brand names like Mounjaro to Ozempic, these medications have been approved by the Food and Drug Administration to lower blood sugar levels. Initially created to treat diabetes, GLP-1s are widely known as “weight loss” drugs.
Since GLP-1s are relatively new drugs, their long-term side effects are unknown. Furthermore, as research on GLP-1 use continues, anesthesia guidelines for patients taking the drug are emerging.
Here’s what CRNAs should know when treating patients on GLP-1s.
GLP-1 Perioperative Guideline Update
In 2023, the American Society of Anesthesiologists (ASA) recommended holding GLP-1s the day prior to or on the day of a patient’s surgery. For those taking weekly dosages, the ASA recommended holding GLP-1s for a week.
Over the past year, guidelines for GLP-1 anesthesia have changed. In a 2024 multi-society update, the ASA advised that most patients may continue the GLP-1 drug before their procedure. However, there are a few exceptions:
- New or early GLP-1 users in the escalation phase
- Patients with gastrointestinal symptoms
- Patients with preexisting conditions like Parkinson’s or other high-risk groups
The ASA also stated that patients receiving higher GLP-1 dosages experienced more side effects and advised them to follow a liquid diet for 24 hours prior to their procedure.
CRNA Perioperative Assessment for GLP-1s
CRNAs must ask their usual screening questions, as well as inquire about the patient’s use of GLP-1s, their dosage, and the duration of their treatment, among other details.
Remember, GLP-1s can delay stomach emptying, so if the patient is considered high risk, the ASA recommends following a liquid diet in the 24 hours before the procedure. In addition, if the patient presents with symptoms such as bloating, abdominal pain, nausea, or vomiting, consideration should be given to delaying elective surgery. Performing a gastric point-of-care ultrasound (POCUS) is also recommended as an added precaution CRNAs can take before treating high-risk patients on GLP-1s.
Intraoperative Management
Delayed stomach emptying caused by the medication may lead to adverse gastrointestinal symptoms, and the residual risk of regurgitation and aspiration can increase the likelihood of fatal aspiration pneumonia.
If you suspect a high-risk patient has a “full stomach” or if the gastric POCUS indicates so, the ANA advises anesthetists to postpone elective procedures if possible. For non-elective “full stomach” procedures, be prepared to enact rapid sequence intubation (RSI) to protect the airways in case of regurgitation.
Postoperative Considerations
ASA med students suggest anesthetists may need to treat patients on GLP-1s with an antiemetic regimen or medication that’s unlikely to cause postoperative nausea and vomiting (PONV).
Most patients can resume GLP-1s as scheduled. Although higher-risk patients or those experiencing gastrointestinal side effects post-op may need to adjust the timing of their next dosage.
For more information, refer to our GLP-1 Agonist and Anesthesia article, which provides a detailed explanation of the mechanism of action and offers comprehensive guidance on anesthesia for GLP-1 patients.
We’re CRNAs ourselves, and we understand the challenge of fitting CRNA continuing education credits into your busy schedule. Whenever you’re ready, we’re here to help.