Did you know?, blue-blooded crabs save millions of lives every year. Dive into this article to explore the science behind endotoxin testing and the ongoing efforts to safeguard both human health and these fascinating creatures.
Okay, we might have gotten off on the wrong foot here. Perhaps “saves” is not quite right. Rather, “prevents death” better captures what it does. We see it both ways, but we’ll let you decide. So read on!
We are willing to bet that many of you have seen them. Perhaps some of you have even touched one (we will make a case that if you do see one, you might consider giving it a helping hand). Acquaint yourself with a 445-million-year-old arthropod, the horseshoe crab, what some refer to as a living fossil.
The horseshoe crab has blue blood, the subject of our musings here, but before that, we must tell you about its eyes, which we mean in the super-plural sense! Adorned on that turtle-like exoskeleton are two compound eyes and seven secondary simple eyes, two of which are on the underside to sense light when it swims—it swims upside down! Its sensitivity to light in the sea’s darkness is one million times greater than that during the day. Oh, and that frightening long “stinger” at the back of the horseshoe crab, it’s not a weapon and nothing to fear. Termed a ‘telson,’ it’s an appendage used like a rudder and for righting itself in the water.
But when it ends up on land to lay eggs by the many thousands in the sand or is washed up and stranded by a wave, being upside down is lethal as it cannot right itself. Hence, you may see signs on the beach with a little photo of them reading: “See ‘em, Flip ‘em!” Why? These living fossils are vital to us as endotoxin sentries.
| Table of Contents
- Colossal Industries Depend on the Horseshoe Crab
- All is Not Well in the Horseshoe Crab Blood Procurement Sector
- Just Use a Chemical Substitute: Ah, Not So Fast
- The Good News (For the Horseshoe Crab) Continues…
Colossal industries depend on the horseshoe crab
Given the size and scope of the pharmaceutical and medical device industries, the word “colossal” seems to fall short of the mark. It’s like saying that the Horseshoe Nebula (1,500 light years away) or the galaxy MACS0647 (13.3 billion light years away) are far from Earth. ‘Far’ does not do justice to the staggering distances.
Worldwide, the production of drugs and devices used in providing care is of just such a scale. Every injectable drug, every parenteral vaccine, and every inserted medical appliance (pacemakers, vascular grafts, stents, implants, catheters, IV access devices, etc.) must be tested for bacterial endotoxin before sterile packaging. This covers drugs or devices that come into contact with intravascular, intralymphatic, intrathecal, or intraocular systems to confirm the absence of endotoxin, thus ensuring their safety. Otherwise, the very life-saving or life-enhancing preparation might prove lethal.
Rabbits were traditionally used as detection sources as they share a dependable and measurable pyrogenic response to endotoxin. But there are a lot of problems with rabbits. Automation is not feasible, and they are difficult to handle because they:
- Must be housed and fed
- Need skilled lab technicians
- Are resource-intensive
Not to mention that pesky concern about animal rights…
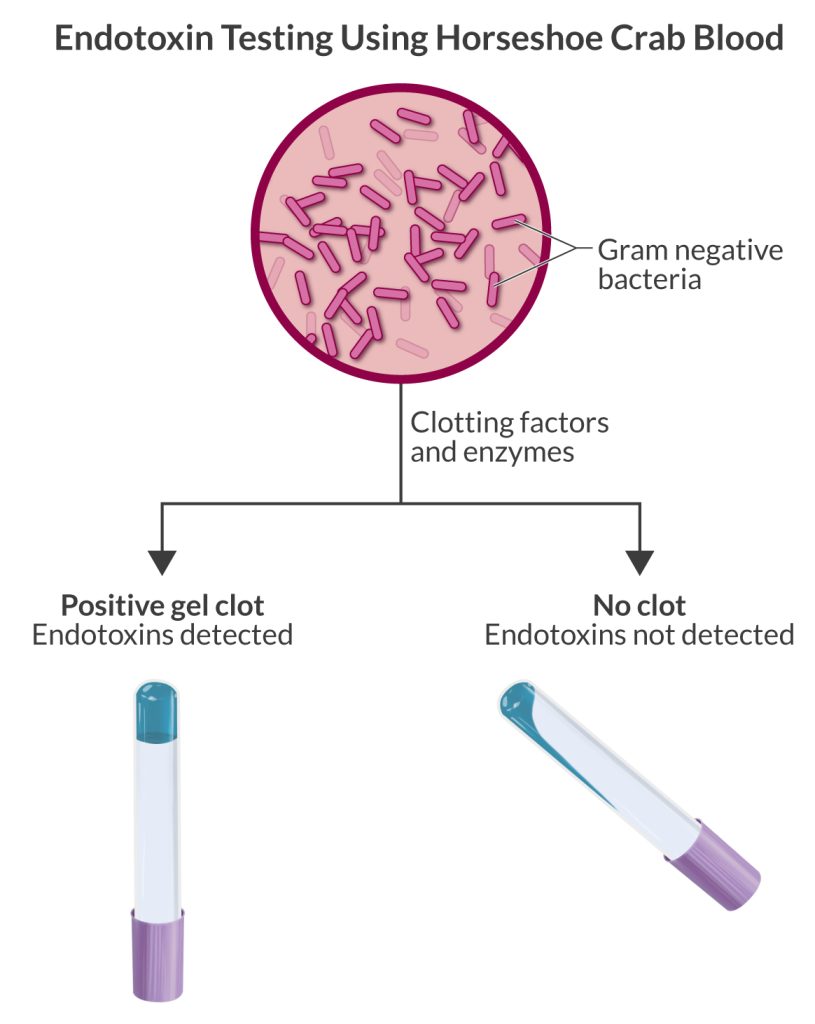
The current standard test uses a constituent obtained from the horseshoe crab’s blue blood—due to the copper-based O2-carrying protein hemocyanin—known as Limulus amebocyte lysate (LAL). Endotoxin encountering the ALA causes coagulation, identifying its presence.
Consequences of endotoxin blood testing from horseshoe crabs
As with most enterprises, obtaining blood from the horseshoe crab has its critics. Horseshoe crabs are plucked from beaches, inlets, and estuaries along the Atlantic coast to obtain blood. They are trucked to a facility and secured side by side on long tables by trained technicians who insert a needle into the horseshoe crab’s heart, removing about half the blood volume over about 6-9 minutes based on algorithms. No one knows the mortality of the horseshoe crabs as it is variably reported, but a 30% death rate is commonly cited, though the fate of those returned to the water is unknowable.
Biomedical blood harvesting facilities have little accountability and virtually no oversight. What’s clear is that horseshoe crab numbers are plummeting with downstream consequences to animals, including two species of sharks and endangered loggerhead turtles dependent on horseshoe crabs for sustenance. Then there is the red knot (which flies from the Arctic to Patagonia – over 10,000 miles and back!), a migratory bird highly dependent on horseshoe crab eggs for a feeding stopover for the long journey. Horseshoe crab eggs are plump, superlative nuggets of nutrition, and red knot numbers are also falling.
Alternative bacterial endotoxin test methods
The complexities of ALA and the unique regulatory demands of the pharmaceutical and medical device industry have proved daunting. The fertile marketplace and economic potential to produce an alternative continue to inspire the quest for an option. One product, recombinant Factor C (rFC), became commercially available in the early 2000s.
Despite the promise, the industry seemed only lukewarmly interested due to limited data, uncertainty of supply, and the absence of regulatory guidance in a domain where federal rules are specific. Things got amped when the FDA issued a window of opportunity by approving rFC. One of the delays in finding a substitute is that LAL is not a drug or a device falling into a unique category of biomedical material.
The good news is that rFC represents a promising modernization of endotoxin testing as it is biotechnologically engineered, pure, and highly specific. It’s easy to produce in unlimited quantities without using animals. And it’s good to know that there are other agents in the pipeline, like recombinant cascade reagent (rCR), to mention just one.
The future of endotoxin testing
Those ‘colossal’ pharmaceutical and medical device industries we mentioned earlier have come a long way since the days of using rabbits for detecting endotoxin. Companies like Eli Lilly and others have responded favorably to an alternative testing approach. The Eli Lilly company recently declared that all new products would undergo rFC testing instead of LAL. They moved the very first rFC-tested drug to market with its release of galcanezumab (EmgalityTM), a migraine drug.
Biomedical engineering and adopting new ideas and interventions is, by nature, a deliberate, systematic, and conservative process. In the case of bacterial endotoxin testing and transitioning away from ALA, high standards of sensitivity will be maintained. At the same time, horseshoe crabs and critters dependent on it will benefit.
As CRNAs ourselves, we understand the challenge of fitting CRNA continuing education credits into your busy schedule. When you’re ready, we’re here to help.